The pharmaceutical industry is defined by its relentless pursuit of innovation, often through strategic partnerships and in-licensing agreements. The process of evaluating potential in-licensing opportunities requires a comprehensive framework that considers multiple facets.
In this 1-page article, Dr Carlos Velez, CELforPharma faculty member of Business Development & Licensing Course For Pharma & Biotech, outlines a general evaluation framework for in-licensing opportunities.
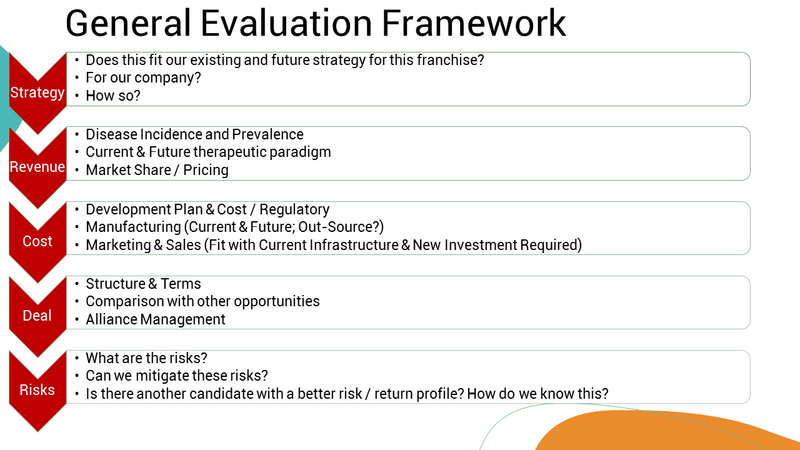
Strategy Alignment:
Central to the evaluation is aligning the potential in-licensing opportunity with the existing and future strategies of both the franchise and/or the company as a whole. Assessing how the candidate fits within the strategic goals, therapeutic focus, and long-term vision is crucial.
Revenue Potential:
To assess the revenue potential of the licensing candidate, you will need to understand disease incidence and prevalence. You will also need to assess how the therapeutic landscape will have evolved by the time the candidate reaches the market to estimate potential market share and pricing.
Cost Considerations:
Evaluation of costs spans across various aspects such as development plans, regulatory requirements, manufacturing feasibility (both present and future), and the marketing and sales infrastructure needed. Balancing these against potential revenue streams is pivotal.
Deal Structure:
The structure and terms of the in-licensing agreement play a pivotal role in determining its attractiveness. A comparative analysis against other potential opportunities helps gauge the strengths and weaknesses, aiding in making informed decisions.
Risk Assessment and Mitigation:
Identifying risks associated with the in-licensing opportunity is essential. Pinpointing strategies to mitigate these risks is critical. Assessing alternative candidates and their risk-to-return ratio provides a broader perspective.
The comprehensive evaluation framework amalgamates these key aspects to paint a holistic picture of the in-licensing opportunity. However, the landscape is not static; constant monitoring and reassessment of these factors are vital as market dynamics, regulatory landscapes, and internal strategies evolve. Successful implementation of this framework demands a collaborative effort, drawing insights from cross-functional teams encompassing R&D, commercial, regulatory, and finance. Their diverse perspectives aid in a more nuanced evaluation, ensuring informed and prudent decision-making.
