By Maaike Addicks, MD, expert-trainer of the Strategic KOL Planning & Engagement course.
A key step in building a strategic Key Opinion Leader (KOL) plan is to identify and map your potential KOLs. There are different ways to map your KOLs, please find here 4 different types:
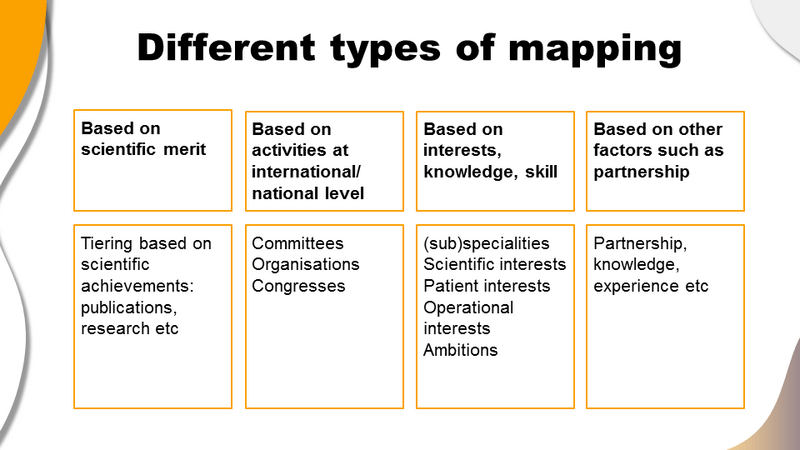
1. Based on scientific merit
This type of KOL mapping focuses on identifying experts based on their scientific contributions and achievements. Factors to consider include publications, research output and recognition within the scientific community. By ranking KOLs according to their scientific achievements, companies can engage with leaders who have a significant impact on medical research and practice.
2. Based on international/national activities
KOLs are also mapped based on their involvement in high-profile activities such as participation in committees, organisations, and congresses at an international or national level. This approach ensures the selection of leaders who are actively shaping policies, guidelines and medical standards on a broader scale, thus impacting a wider audience.
3. Based on interests, knowledge and skills
Another approach is to map KOLs according to their specific interests, knowledge, and skills. This includes identifying specialists in different subspecialties, those with particular scientific or patient interests, and those with operational expertise or ambitions. By targeting KOLs with aligned interests, pharma can foster more relevant and productive collaborations and understanding the KOLs at this level will allow you to fully meet their needs.
4. Based on other factors such as partnership
KOL mapping can also be driven by factors such as existing partnerships, and experience. This approach considers the history of collaboration and the depth of expertise a KOL brings to the table.
The type of mapping you choose will depend on your company's policies, the goals set in your medical plan and where you are in the product lifecycle.
Continue your learning from Maaike
If you’d like to learn more from Maaike, CELforPharma also offers a 1-day, hands-on course where you'll learn how to:
- Identify, prioritise, and map the right type of KOLs for your medical objectives
- Build engagement plans that evolve with your product’s lifecycle
- Create mutually valuable, trust-based partnerships with experts
- Strengthen the quality, structure, and internal alignment of your KOL activities
Don’t miss the latest insights from our expert faculty
Subscribe to our newsletter to:
- Stay on top of the latest expert insights
- Receive invitations to upcoming educational webinars
- Get updates on our courses and training programmes
