By Dr. Nick Proctor, expert-trainer of the Understanding Pharma Market Access & Payers in Europe course.
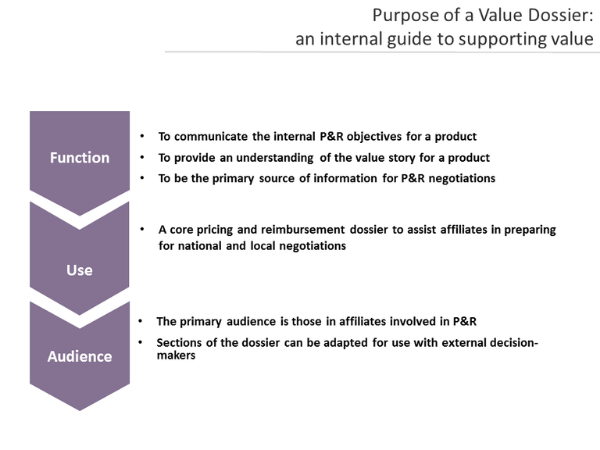
The Value Dossier is the core document produced by global market access teams for use by national affiliate sales and marketing organisations. It is typically delivered to the affiliate market access colleagues some months before launch and is a distillation of the available data on current treatments and the value story of the brand or indication to support submissions and interactions with payers at the country level.
The development of the Value Dossier is a multidisciplinary task, with critical inputs from health outcomes, medical affairs, clinical development and marketing but it is owned overall by the global market access team. While its function is primarily to support market access teams, it is critical that the parallel development of clinical messages is consistent with those developed for payers. In the Understanding Pharma Market Access & Payers in Europe course, we explore the consequences of misaligned stakeholder messages and how to avoid.
The structure of the value dossier varies slightly by company and product but the themes throughout should be focused to support the consistent brand value story, composed of multiple value messages which have been developed and tested with payer primary research. Country and payer-level tailoring is usually required to refine the messages and ensure the supportive evidence is relevant for the stakeholders.
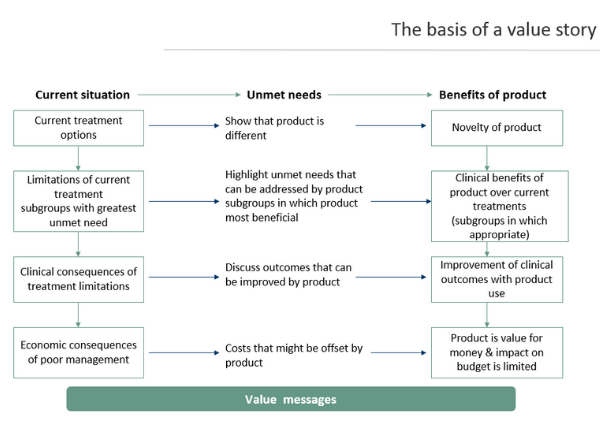
The development of successful global value messages often follows this structure which results in a consistent and multi-level set of messages for payer stakeholders. These stakeholders are commonly defined by archetypes by global teams for ease of adaptation to country level.
Since most payers are generalists and not familiar with the detail of therapy areas, it is an important first step to describe the current treatment options and any clinical and economic challenges associated with them. This is a critical step in establishing how a novel product can add value to the health system which any payer is responsible for. The following steps deal with product attributes and how they are focused towards showing the additional benefit beyond that of existing treatment options, both clinical and economic.
During our Understanding Pharma Market Access & Payers in Europe course, we discuss the organisational and process needs required to construct effective value stories and work through the development of winning value communication with participants.
